 The IML provides a broad range of state-of-the-art immunologic monitoring assays, and also designs, evaluates, and standardizes new products and assays, to support clinical trials of novel biological therapies for cancer patients. The IML has an independently-monitored and extensive quality control (QC) and quality assurance (QA) program to ensure the validity of test results and safety/quality of therapeutic products. The IML is CAP inspected and participates in several external proficiency panels for assays. The IML also serves as the Central Immunology Laboratory for the ECOG-ACRIN cooperative oncology group.
The IML provides a broad range of state-of-the-art immunologic monitoring assays, and also designs, evaluates, and standardizes new products and assays, to support clinical trials of novel biological therapies for cancer patients. The IML has an independently-monitored and extensive quality control (QC) and quality assurance (QA) program to ensure the validity of test results and safety/quality of therapeutic products. The IML is CAP inspected and participates in several external proficiency panels for assays. The IML also serves as the Central Immunology Laboratory for the ECOG-ACRIN cooperative oncology group.
Organization of the IML
To meet widely diverse service demands, the IML is organized into seven areas:
- Specimen intake/processing
- Cytokine assessment
- Cytotoxicity assessment
- Flow cytometry
- Tissue culture
- Data entry and analysis
- Research and development
Assays Available in the IML
The laboratory now performs over 80,000 assays per year. These can be performed in real time on fresh blood, while serial monitoring of samples over multiple time points are batched and tested in one assay to minimize inter-assay variability.
Tests are routinely available for assessing lymphocyte functions, including:
- Cytotoxicity: natural killer (NK) cells; cytotoxic T lymphocytes (CTL); lymphokine-activated killer (LAK) cells; and antibody-dependent cell-mediated cytotoxicity (ADCC)
- Proliferation microassays (responses to lectins, antigens, superantigens, mixed lymphocyte cultures, mixed lymphocyte-tumor cell cultures)
- Cytokine production and serum levels by bioassays and immunoassays (ELISA and multiplexing Luminex technology) for a variety of cytokines, chemokines, growth factors and growth factor receptors
- Single-cell multi-cytokine assessment
- Assays for signaling defects, e.g., expression of the zeta chain in T cells or NKG2D in NK cells
- A range of assays for apoptosis (Annexin V binding, caspase activation)
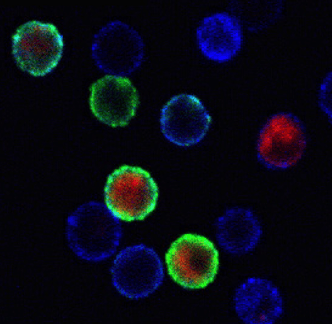
- Multi-color flow cytometry analysis for an extensive array of mononuclear cell (MNC) surface markers as well as intracellular proteins in permeabilized MNC or tissue cells (including signal transduction pathway molecules by phospho-protein flow cytometry)
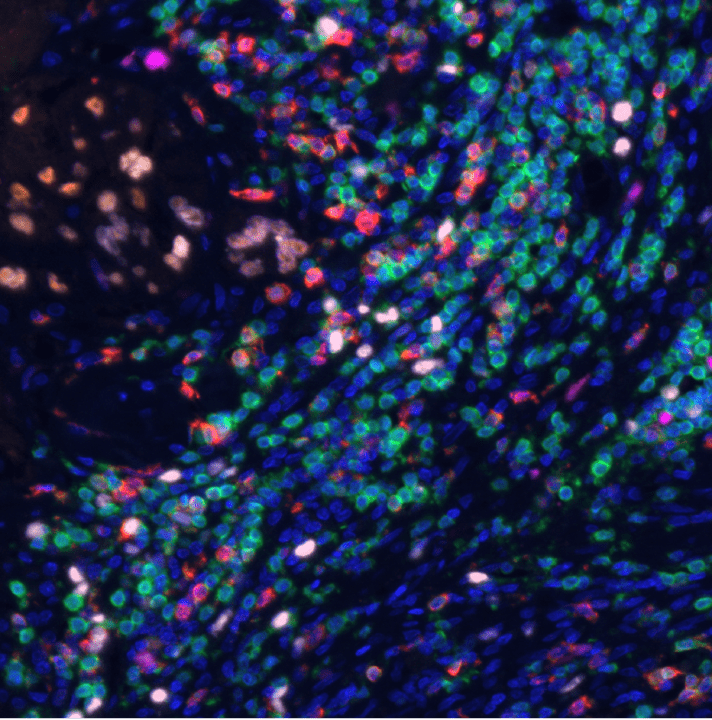
- Multispectral imaging of cells in tissues by Vectra picture
Absolute numbers of lymphocyte subsets are determined by a single-platform flow-based method. Assays for antibody responses to proteins and viruses are performed. Assays for monocyte and granulocyte functions include cytolysis, H2O2 production, and production of monokines. Single-cell assays routinely available for quantification of antigen-specific T cells include ELISPOT for IFN-γ, IL-2, IL-5, and granzyme B; cytokine flow cytometry (CFC); and tetramer binding.
To monitor patients treated with anti-tumor vaccines, including tumor peptides, dendritic cells +/- peptides, genetically-modified tumor cells, etc., the IML has developed multiple methodological approaches and has available a panel of assays which includes:
- Flow cytometry screen for MHC class I antigens A2 and DR4
- Mixed lymphocyte–tumor cultures (MLTC) to generate T cells from peripheral blood, tumor- infiltrating lymphocyte (TIL), or other body fluids and sites
- Cytotoxicity assays of various types, including standard 4h 51Cr-release assays against tumor cell targets +/- blocking with mAbs, flow-based cytotoxicity, and CD107a release
- Proliferation assays with T cells responding to antigens (3H-thymidine uptake and CFSE dilution), including multiple rounds of in vitro sensitization (IVS)
- Phenotypic characterization of CD4+ and CD8+ T, DC, NK, NK/T, B, MDSC and Treg cells by multi-color flow cytometry
- Single and multiplexed cytokine assays for production and body fluid levels, including standardized cytokine, chemokine, and growth factor measures
 ELISPOT, CFC, and multimer assays for determining changes in the frequency of antigen-specific T cells capable of binding or functionally responding to vaccinating peptides or antigens by secretion (ELISPOT) or by expression of cytokines (CFC)
ELISPOT, CFC, and multimer assays for determining changes in the frequency of antigen-specific T cells capable of binding or functionally responding to vaccinating peptides or antigens by secretion (ELISPOT) or by expression of cytokines (CFC)- Evaluation of expression of signaling molecules in T cells by intracytoplasmic staining
- Determinations of the frequency of T cells binding Annexin V, and thus undergoing apoptosis in the peripheral circulation, before and after vaccination therapy
- Titers of antibodies to a selected number of antigens by ELISA or flow cytometry
The Facility has the ability to process large volumes of blood (up to 200 ml per patient) as well as leukapheresis products; it cryopreserves (in a rate–controlled manner) and banks patients’ cells for later simultaneous testing of sequentially–collected specimens.
The laboratory is responsible for shipping samples collected from patients enrolled at other clinical sites to UPMC Hillman Cancer Center, or from those on Hillman protocols for assays not performed at this institution and for ECOG-ACRIN–sponsored protocols.
In collaboration with the Hillman Biostatistics Facility, the IML has developed a number of programs for data analysis, including an improved lytic unit transformation program as well as curve–fitting programs for cytokine assays. A jointly written program provides for data verification and checking of outlier results prior to final data analysis for each protocol. The data generated by the IML are computerized, checked for accuracy, and made available to biostatisticians for analysis.


