Researchers from UPMC Hillman Cancer Center and colleagues published their results from the CAMILLA trial showing that combining cabozantinib and durvalumab in patients with chemotherapy-refractory colorectal cancer is a promising treatment, potentially expanding treatment options for patients with microsatellite stable advanced colorectal cancer.

Anwaar Saeed, MD
“Colorectal cancer is the fourth most common cause of new cancer diagnosis and the second leading cause of cancer death in the United States,” says lead author Anwaar Saeed, MD, Section Chief of Gastrointestinal Medical Oncology and the Director of the Gastrointestinal Disease Center. “Though outcomes have improved for all stages combined, advanced and metastatic colorectal cancer still have a poor prognosis.”
First-line treatment for advanced colorectal cancer has been the same for decades, options for second-line treatment are still narrow, and a very small percentage of patients have newer targeted or immunotherapy treatment options.
Once patients require a third-line treatment, the majority have tumors with characteristics that do not respond to immune checkpoint inhibitors (ICI), making immunotherapeutic treatments ineffective. By developing treatment strategies that modulate the tumor microenvironment to be more receptive to ICI, more patients could benefit from immunotherapy. In this study, researchers show that cabozantinib plus ICI is a promising strategy for patients who typically do not respond to single-agent ICIs.
Cabozantinib is a multi-target tyrosine kinase inhibitor that has the potential to harbor immunomodulatory effects that impede the immune suppressive tumor microenvironment. Pre-clinical studies and early phase trials showed evidence of cabozantinib’s effectiveness, triggering the ongoing CAMILLA trial that combines cabozantinib with durvalumab.
Among the 29 patients enrolled on the colorectal cancer cohort, authors report an objective response rate of 27.6% and a median overall survival of 9.1 months. Notably, among RAS wildtype patients, the objective response rate reached 50% and median overall survival of 21.5 months, which demonstrates the potential influence of RAS mutational status possibly contributing to deeper and more durable responses to cabozantinib plus durvalumab.
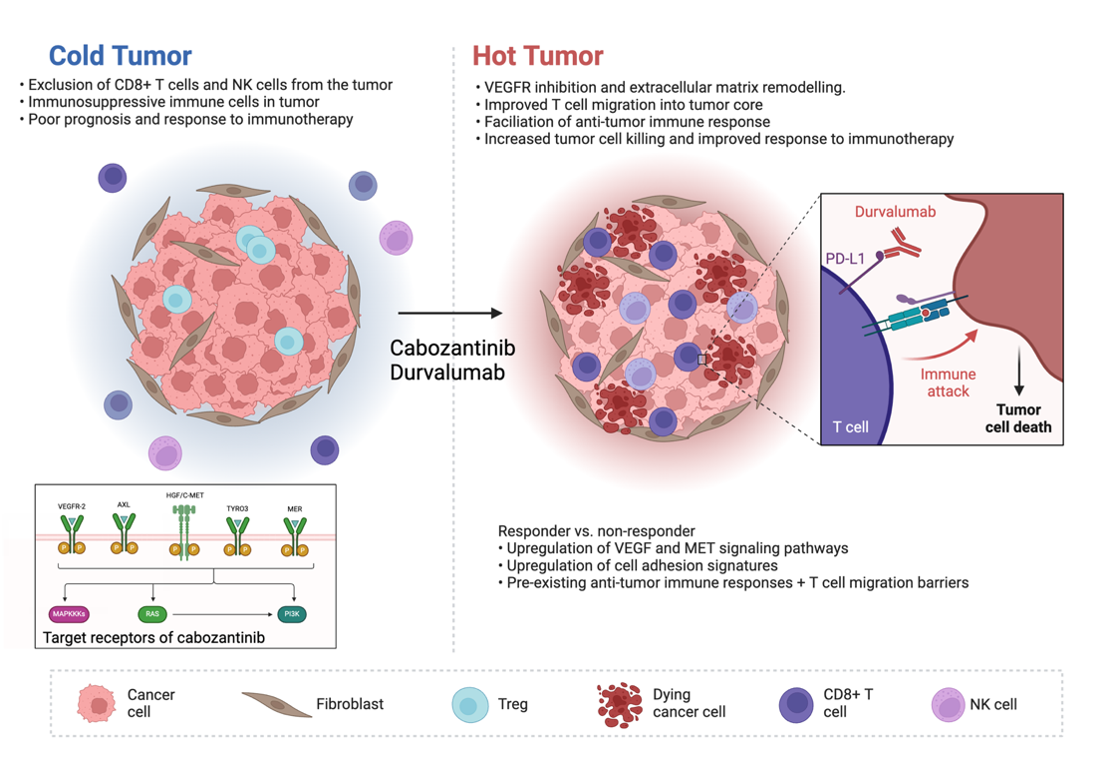
Figure 1
Additionally, the published CAMILLA data supports that cabozantinib modulates the tumor microenvironment and improves checkpoint inhibitor response. Molecular analysis of trial patient’s tumor samples suggests that cabozantinib leads to VEGFR2 and MET signaling pathway inhibition and extracellular matrix remodeling, which improves T cell migration into the tumor core and facilitates the durvalumab anti-tumor immune response (Figure 1).
The CAMILLA trial’s promising results have led to the phase III STELLAR 303 trial investigating the combination of zanzalintinib — a next generation multi-kinase inhibitor very similar to cabozantinib — and atezolizumab. Led by Dr. Saeed, this trial will help guide further innovative strategies for combinational ICI-based treatments to challenge the status quo of colorectal cancer immunotherapy options.
Written by: Anwaar Saeed and Annaliese Daniels
Reviewed by: Anwaar Saeed


