Project Co-Leaders:
Greg M. Delgoffe, PhD (Basic Co-Leader)
Dan P. Zandberg, MD (Clinical Co-Leader)
This SPORE project is a continuation of Project 1 from the previous cycle of the SPORE grant, which evolved from a successful Developmental Research Program project. It is testing the hypothesis that tumor hypoxia represses T cell function in HNSCC and that the mechanism leading to this repression includes targetable markers of anti-PD-1 resistance. The project builds on published data showing that the metabolic landscape of the tumor microenvironment (TME) has a profound effect on patient response to checkpoint blockade immunotherapy and that inhibitors of oxidative phosphorylation can reduce hypoxia and improve anti-PD-1 responses in animal models. The three aims of Project 1 are shown in the accompanying schematic.
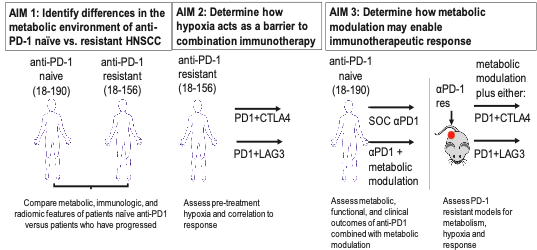
This project will leverage biospecimens and computed tomography (CT) images made available by the Tissue/Pathology/Imaging Core and include two investigator-initiated trials conducted in patients with recurrent or metastatic HNSCC who are either naïve to anti-PD-1 immunotherapy (NCT04114136) or have progressed while receiving anti-PD-1 immunotherapy (NCT04326257). The trial in immunotherapy naïve patients is determining the effects of combining anti-PD-1 monotherapy with metabolism-altering drugs used to treat type 2 diabetes. It is comparing anti-PD-1 monotherapy with nivolumab to nivolumab + metformin and to nivolumab + rosiglitazone. The trial in anti-PD-1 resistant patients is determining whether addition of a second immunotherapy agent, which targets a different inhibitory receptor, can overcome innate or acquired anti-PD1 resistance. It is comparing the combination of nivolumab plus ipilimumab (anti-CTLA-4 agent) to nivolumab plus relatlimab (anti-LAG-3 agent).
The preclinical and translational studies of Project 1 have been designed to answer the following questions pertaining to anti-PD1 immunotherapy resistance:
- What is the relationship between anti-PD-1 resistance, tumor metabolism, and hypoxia in HNSCC?
- Does hypoxia promote resistance to combinatorial immunotherapy in HNSCC?
- Can metabolically targeted therapy be combined with immunotherapy to overcome anti-PD1 resistance in HNSCC?
Successful completion of Project 1 will provide a predictive biomarker for assessing whether a patient is resistant to anti-PD-1 immunotherapy, which is now standard of care for advanced HNSCC, and may lead to larger clinical trials of combinatorial therapy.


