Project Co-Leaders:
Tullia Bruno, PhD (Basic Co-Leader)
Dario Vignali, PhD (Basic Co-Leader)
Dan P. Zandberg, MD (Clinical Co-Leader)
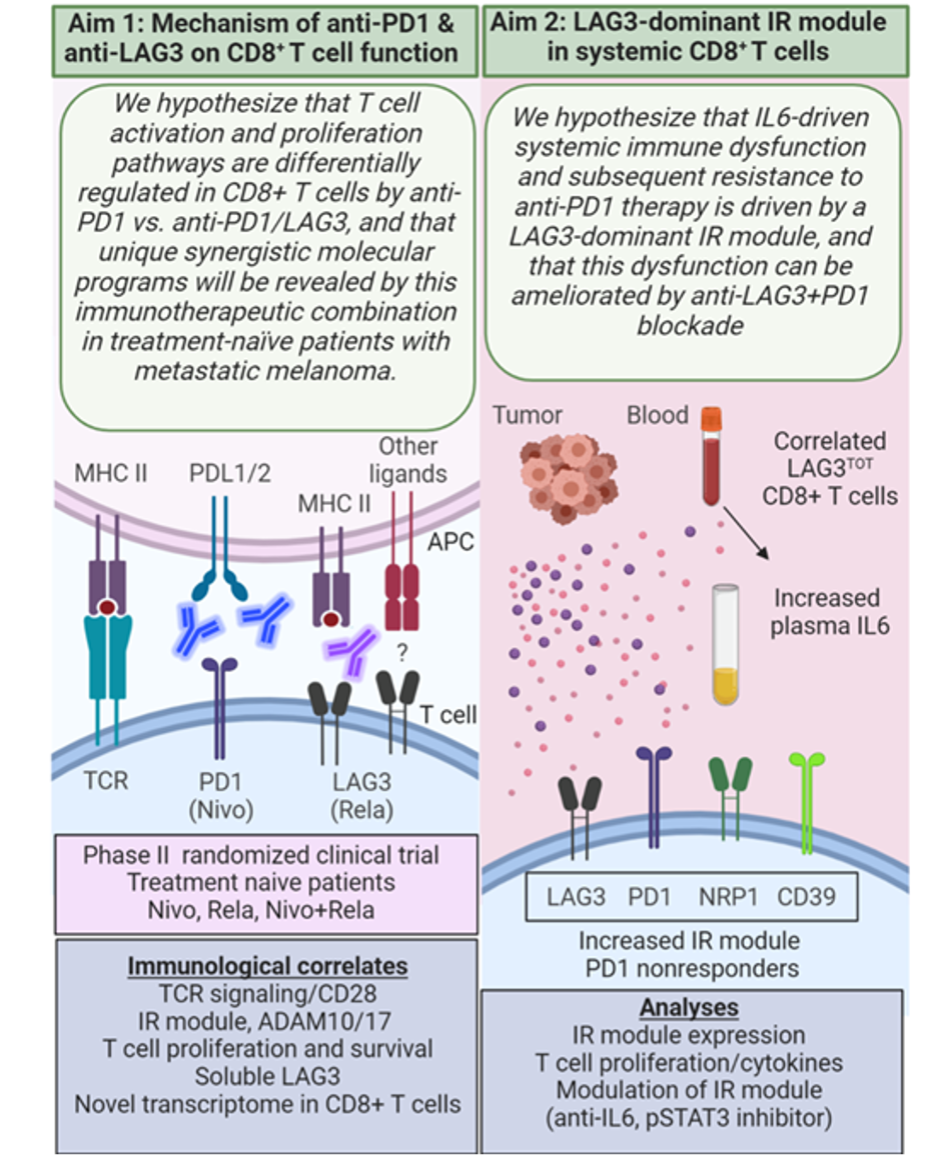 This new SPORE project evolved from a successful Developmental Research Program project funded during the prior SPORE grant cycle and leverages a recent clinical trial of combinatorial immunotherapy (NCT01968109) that included patients with HNSCC. It is addressing the overarching hypothesis that LAG3/PD1 dual blockade synergizes to promote CD8+ T cell function in the tumor and peripheral blood of HNSCC patients, and that a LAG3-dominant inhibitory receptor module in peripheral CD8+ T cells downregulates T cell function and ultimately patient response to PD1-targeted therapy.
This new SPORE project evolved from a successful Developmental Research Program project funded during the prior SPORE grant cycle and leverages a recent clinical trial of combinatorial immunotherapy (NCT01968109) that included patients with HNSCC. It is addressing the overarching hypothesis that LAG3/PD1 dual blockade synergizes to promote CD8+ T cell function in the tumor and peripheral blood of HNSCC patients, and that a LAG3-dominant inhibitory receptor module in peripheral CD8+ T cells downregulates T cell function and ultimately patient response to PD1-targeted therapy.
The two aims of Project 3 are shown in the accompanying schematic (click for full size). The project includes a new SPORE-specific neoadjuvant window trial in which treatment-naïve locally advanced HNSCC patients are being randomized to receive either nivolumab (anti-PD-1) monotherapy or the combination of nivolumab + relatlimab (anti-LAG-3) followed by surgery, and utilizes new single-cell, multi-parametric technologies made available by the Tissue/Histology/Imaging Core to characterize immune cell populations in the TME and periphery. The project’s studies have been designed to address two key questions:
- What is the mechanism of anti-PD-1 and anti-LAG3 on the anti-tumor function of CD8+ T cells?
- Does IL6 drive a LAG3-dominant IR module in peripheral CD8+ T cells and does it predict responsiveness to immunotherapy?
Successful completion of Project 3 will define biomarkers of response and resistance to nivolumab and nivolumab + relatlimab and identify a patient population that will optimally benefit from inclusion of LAG3-based therapies.


